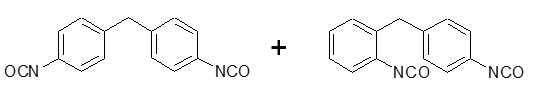3. MDI Products
MDI Production Process
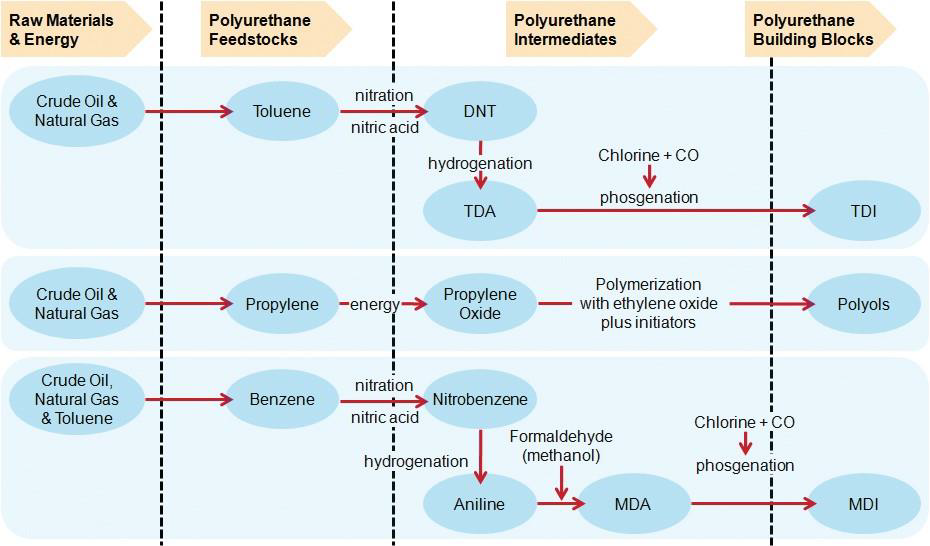
In the MDI process, aniline is condensed with formaldehyde to produce methylenedianiline (MDA), and MDA is reacted with phosgene to form MDI. The flexibility of the BASF process makes it possible to control and modify the properties of a broad range of MDI products. This process is designed with the flexibility that allows BASF to be a reliable source of MDI for the polyurethane industry. Figure 2 gives a summary of the MDI, TDI, and polyether polyol production processes.
Figure 2. Process summary for the production of polyurethane building blocks.
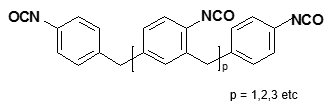
Figure 3 shows the molecular structure of the primary MDI molecules: Polymeric MDI (PMDI) and Monomeric MDI (MMDI).
Monomeric MDI
Polymeric MDI
Figure 3. Molecular structure of monomeric and polymeric MDI.
MDI Product Family, Properties, and Applications
MDI is available in three basic types of compositions: PMDI, MMDI, and Modified MDI. MDI and its modified forms are reactive chemicals supplied as liquids or solids. In combination with polyols (polyester or polyether), they are used for the manufacture of cellular (foamed) and non-cellular (compact) polyurethane polymers including textiles, coatings, adhesives, sealants, and elastomers (CASE). BASF MDI Products can be used in many applications
within CASE and other markets.
PMDI
PMDI is a brown liquid, stable over a wide range of temperatures. It contains a mixture of polyaromatic isocyanates including 2-ring (MMDI), 3-ring, and higher molecular weight species. As the amount of 3-ring and higher molecular weight species increase, the functionality and viscosity of the PMDI will also increase. PMDI products have a density of approximately 1.23 g/cm3 at 77ºF (25ºC). PMDI starts to decompose at temperatures above 446°F (230°C) with the evolution of carbon dioxide (CO2). At 77°F (25°C), it has a low vapor pressure of 10-5 mbar (7.5X10-6 mm Hg). PMDI is considered to have a low flammability risk due to its high flash point of over 392ºF (200ºC).
The thermal conductivity of PMDI decreases with temperature until approximately 122 to 140ºF (50 to 60ºC), where it increases with temperature (see Table 1). This behavior is somewhat atypical for liquids and is, in part, due to trimerization at elevated temperatures. The dielectric constant of PMDI is 7.3 at 104ºF (40ºC) and 6.5 at 176ºF (80ºC). The conductivity is 1.6 micro-mho at 200 cPs and 1.2 micro-mho at 700 cPs. The coefficient of thermal expansion for PMDI is 0.0008/ºC. Specific heat information for PMDI is listed in Table 2.
Table 1. PMDI thermal conductivity values.
| Temperature (°C) | Thermal Conductivity (W cm-1 K-1) |
| 22 |
0.00164 |
| 48 | 0.00139 |
| 57 | 0.00155 |
Table 2. PMDI specific heat values.
| Temperature (°C) | Specific Heat (J g-1 °C-1) |
| 25 |
1.469± 0.100 |
| 50 | 1.512± 0.100 |
| 100 | 1.577± 0.070 |
| 150 | 1.675± 0.060 |
PMDI is used in producing high resilience flexible, rigid, and packaging polyurethane foams and in a number of non-foam applications such as carpet backing, adhesives, composite wood binder, plywood patching compounds, and foundry core binders. BASF PMDI products are typically bulk-produced in Geismar, Louisiana.
PMDI Blends and Derivatives
Starting with core PMDI and MMDI products, BASF developed a line of isocyanate blends and derivatives that can deliver the custom properties demanded by specific applications. For example, the 2,4’-MDI enhanced PMDIs are
selected for floor coatings that require extra working time, but ultimately maintain cross-linking potential. BASF’s PMDI blends and derivatives are available in drums, totes, and bulk quantities.
MMDI
MMDI is a purified material distilled from a PMDI mixture. MMDI refers to the 2,4’ and 4,4’-isomers of MDI. Pure 4,4’-MDI is a crystalline solid at room temperature. BASF sells frozen (below 32ºF or 0ºC) drums of 4,4’-MDI as Lupranate® M and MS isocyanates. MMDI is a solid with a melting point of approximately 101.3°F (38.5°C) and a boiling point of 410°F (210°C) at 7 mbar (5 mm Hg). Above 113ºF (45ºC), pure 4,4’-MDI can be shipped and kept as a liquid for up to fourteen days. It starts to decompose at approximately 446°F (230°C). At 77°F (25°C), it has a low vapor pressure of 10-5 mbar (7.5X10-6 mm Hg). MMDI has a density of 1.20 g/cm3 and specific heat of 1.39 J/g/ºC at 40ºC (104ºF). MMDI is considered to have a low flammability risk due to its high flash point of 392ºF (200ºC). Increasing amounts of 2,4’-MDI and 3-ring or greater PMDI into the 4,4’-MDI, results in a mixture that is
a liquid at room temperature.
MMDI is used in a multitude of thermoplastic and cast elastomer applications and is the starting material for a variety of modified MDI products. It is also used to prepare coatings, adhesives, sealants, and synthetic fibers. Our product catalogue displays the several variations in isomer distribution and stabilizer package that are available in the MMDI product line.
Carbodiimide Modified MDI
Given the handling and storage difficulties associated with pure 4,4’-MDI, BASF often uses carbodiimide chemistry to modify and stabilize the MMDI. The carbodiimide-modified isocyanates are liquids that are stable and clear at room temperature. A portion of the MMDI is reacted to yield a carbodiimide-modified isocyanate with a free-NCO weight between 29.2% and 29.5%. The carbodiimide-modification leads to the formation of a trifunctional uretonimine species, by reaction with some of the remaining difunctional MMDI. Therefore, the commercial carbodiimide-modified isocyanates are usually listed as 2.1 functional. Carbodiimide Modified MDI products are used in reaction injection molded (RIM) polyurethane automotive body parts, microcellular elastomers, integral skin foams, flexible foams, adhesives, coatings, sealants, and two-component cast elastomers.
MDI Prepolymers (Quasi-)
BASF offers MDI prepolymers (quasi-) based on the reaction of MMDI, PMDI, and/or carbodiimide modified MDI with a variety of resins, including polyether, polyester, PolyTHF®, and others. A prepolymer results from the reaction of excess MMDI with a hydroxyl terminated resin. The exact term, quasi-prepolymer, refers to a reaction product that is a mixture of prepolymer (capped-resin) and excess MMDI.
Properties of MDI
MDI is denser than water and will therefore sink to the bottom of water-filled containers. Although it reacts with water, the rate of reaction is very slow at temperatures below 122°F (50°C). At higher temperatures or in the presence of catalysts/basic materials, the reaction becomes progressively more vigorous and can become violent. The reaction of MDI with water liberates CO2 gas and forms insoluble polyurea compounds. These polyurea products often take the form of globules with liquid isocyanate and CO2 trapped inside. These globules can float due to the trapped CO2. The trapped isocyanate will react with water over time.
MDI reacts with acids, alcohols, basic materials (e.g., sodium hydroxide, ammonia, and amines), magnesium and aluminum (and their alloys), metal salts (e.g., tin, iron, aluminum, and zinc chlorides), strong oxidizing agents (e.g., bleach and chlorine), polyols, and water (CPI, July 2003). These reactions may be violent, generating heat, which can result in an increased evolution of isocyanate vapor and/or a buildup of pressure due to CO2 evolution within closed containers.
MDI is not generally corrosive towards metals or other materials at room temperature, but the presence of a small amount of acidity in PMDI can produce some corrosion with copper alloys and aluminum. Copper, zinc, or their alloys must be avoided as they may cause product deterioration. Carbon steel is the recommended material for MDI containers. Stainless steel is the recommended material of construction for pumps, discharge pipelines, and valves. MDI will attack and embrittle many plastic and rubber materials which may cause these materials to crack over time. Flex Hose must be Teflon®-lined or specially sold and labeled for isocyanate handling. Table 3 gives molecular formulas and constants for the three types of MDI. The single MDI forms have been characterized by several identity numbers as made up in this table.
Table 3. MDI molecular formulas and constants.
| Species | Formula | Molecular Weight | NCO-content (wt %) |
| Monomeric MDI | C15H10N2O2 | 250.26 | 33.50% |
| Prepolymers Modified MDIs |
varies | varies | varies |
| Polymeric MDI | varies | approximately 340 | approximately 31% |
| Reference Numbers | |||
| Chemical Abstracts | 2,4'-MDI: 5873-54-1 | ||
| 4,4'-MDI: 101-68-8 | |||
| 2,2'-MDI: 2536-05-2 | |||
| polymeric MDI: 9016-87-9 | |||
| non-isomer specific: 26447-40-5 | |||
| United Nations | 4,4'-MDI containing compositions: unregulated | ||
| United States | 4,4'-MDI containing compositions greater than 5,000 pounds in a single container; NA 3082 | ||
| EEC-Number | PMDI: 615-005-01-6 | ||